NORTHFIELD, Ill. (March 13, 2024) — The College of American Pathologists (CAP) convened a multi-disciplinary panel of experts to develop evidence-based recommendations on appropriate testing for amyloidosis, a rare disease characterized by abnormal protein deposits in various organs, hindering proper organ function. —The draft statements are available for public comment through April 3, 2024 on cap.org.
New treatments are available for systemic amyloidosis that contribute to an improved quality of life and even potential cure for patients; however, there is variability and inconsistency among pathologists and laboratories in their approach to amyloidosis workup and diagnosis.
“Proper evaluation and testing for amyloidosis are crucial for accurate diagnosis, assessing organ involvement, planning treatment, monitoring response to therapy, and contributing to clinical research efforts,” explains guideline co-chair Dylan V. Miller, MD, FCAP. “This process ensures tailored care, early detection, and ongoing optimization of treatment strategies for individuals with amyloidosis,” adds co-chair Billie S. Fyfe-Kirschner, MD, FCAP.
The forthcoming evidence-based guideline includes seven draft statements that address:
- Guidance on evaluation of specimen types likely to be biopsied as surrogate sites (separate from the target organ involvement) received for detection of systemic amyloidosis.
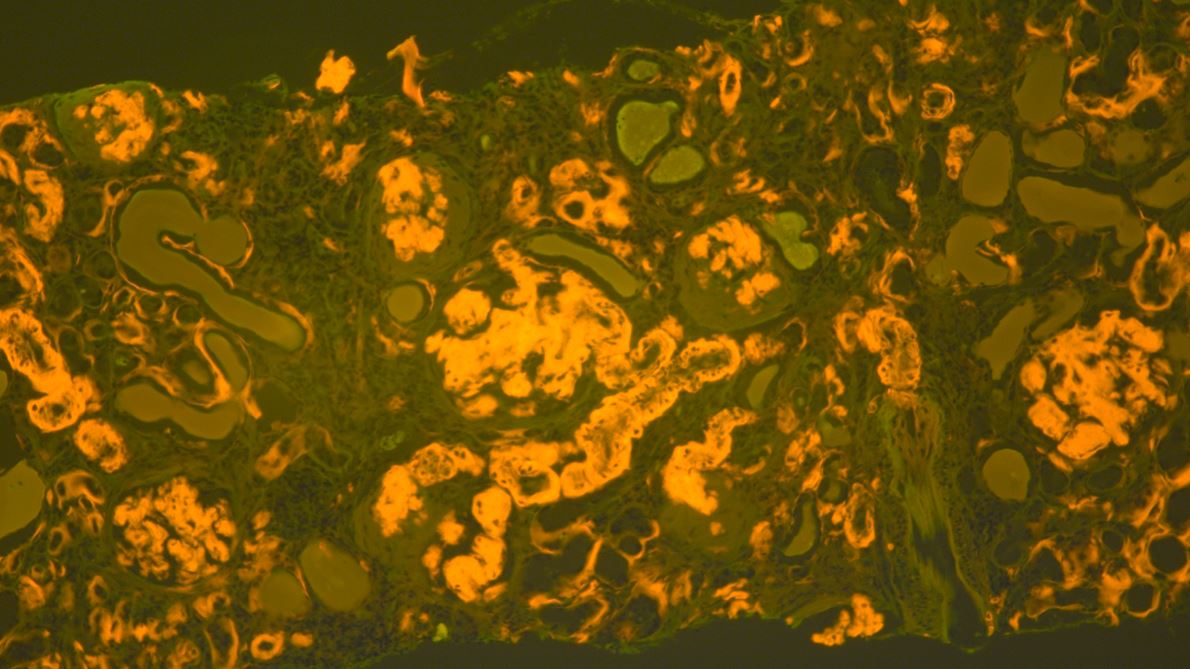
- Method(s) for detecting amyloid and determining the fibril protein type.
- The use of fluorescence microscopy for the detection of amyloid.
- Use of cytology specimens to detect amyloid.
General and specialty surgeons, oncologists, pathologists, histotechnologists, cardiologists, hematologists, nurses, allied health professionals, hospital or laboratory administrators, patient advocacy group representatives, and patients are encouraged to provide feedback on these draft statements.
Following the open comment period, the guideline authors will consider all feedback to finalize the statements. The final statements will appear in the guideline manuscript and will be available at no cost.
To read and comment on the draft statements, visit cap.org between March 13, 2024 and April 3, 2024.
About the College of American Pathologists
The CAP Pathology and Laboratory Quality Center for Evidence-based Guidelines develops evidence-based guidelines related to the practice of pathology and laboratory medicine. Through this work, the CAP and its members continually improve the quality of diagnostic medicine and patient outcomes.
As the world’s largest organization of board-certified pathologists and leading provider of laboratory accreditation and proficiency testing programs, the College of American Pathologists (CAP) serves patients, pathologists, and the public by fostering and advocating excellence in the practice of pathology and laboratory medicine worldwide. For more information, visit the CAP Newsroom, CAP.org and yourpathologist.org to watch pathologists at work and see the stories of the patients who trust them with their care.
###