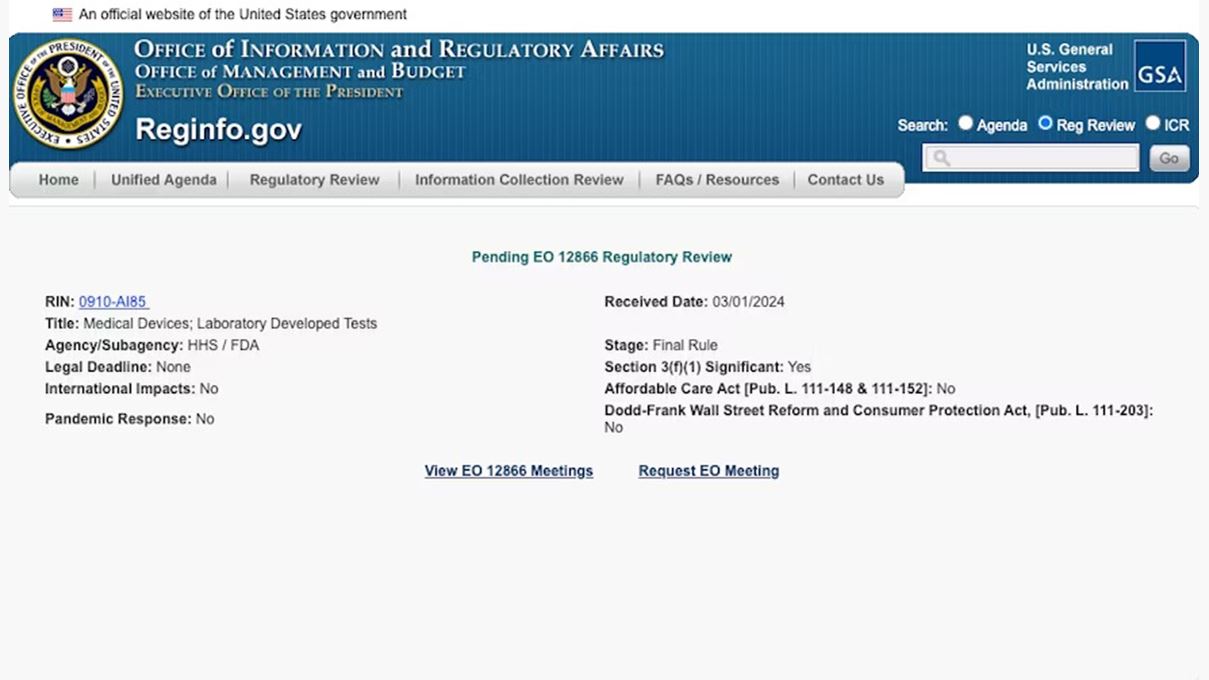
Meanwhile, Archives of Pathology and Laboratory Medicine's February edition took a critical look at an important laboratory quality step, proficiency testing (PT). The article focused on next-generation sequencing (NGS) assays, a class of LDTs in practice in higher volume in recent years.
For the full article click here: FDA LDT oversight battle: Are we close?